Originally posted by Lokiel, Sun Sep 24, 2017 5:15 pmOriginally posted by Lokiel, Mon Mar 28, 2016 4:08 pm[Thanks Mark, I've wanted to do this for so long but knew I needed a few days to do it the first time.
Damn, just noticed that I spelled "beautifully" wrong in an image above AND cut&pasted it into the next image

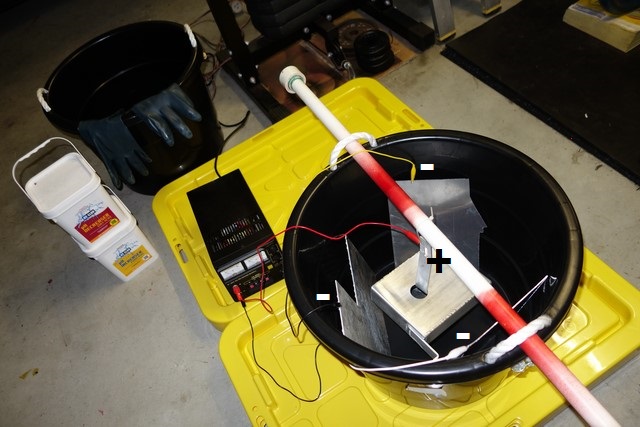
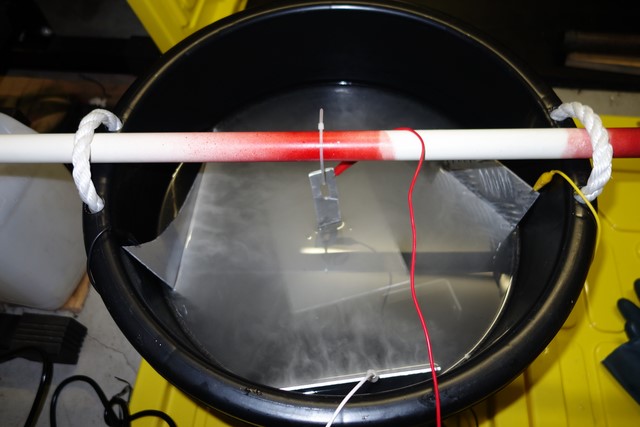
While I had the anodising stuff all set up, I decided to anodise my D514A coil brackets too.

The surface area is much less than the coolant overflow tank so only 2 cathodes were needed:

No blemishes on these but not quite as black as the coolant tank. Maybe it was due to the aluminium composition being different or the electrolyte was too weak after anodising the coolant tank twice (I cranked the amperage up to 4.5amps to "maintain the fizz"):


 Originally posted by The American, Mon Mar 28, 2016 4:39 pm[
Originally posted by The American, Mon Mar 28, 2016 4:39 pm[Very cool!
Originally posted by Roadrunner, Mon Mar 28, 2016 8:19 pm[That's come up great and should be much easier to keep clean compared to black spray paint.
Great stuff!
Are you going to anodise the airbox now?
 Originally posted by Lokiel, Mon Mar 28, 2016 9:20 pm[
Originally posted by Lokiel, Mon Mar 28, 2016 9:20 pm[Roadrunner wrote::
Are you going to anodise the airbox now?

Yeah, I really like the look of the black anodised aluminium and the fact that it's harder to scratch than regular aluminum - or paint

The airbox is about 4-5x the size of the coolant tank.
I'm going to need a 50L rectangular tub for anodising since it must also house the cathodes.
I can probably use a plastic tub with aquarium heaters for the dyeing phase to keep it at 42-43*C.
Finding a pot/tub large enough for the Sealing phase (you need to boil the crap out of the part for ~15 minutes) will be harder.
I suspect I'll need to re-make the airbox wall when I install the TSE manifold since the turbo will sit much higher so the intake hole will be in the wrong position.
Originally posted by Roadrunner, Mon Mar 28, 2016 10:22 pm[Mix it in a 40gal drum. Then your neighbours will really start to get worried lol
Originally posted by ManiacLachy, Tue Mar 29, 2016 7:28 am[That is very cool, and the finish is beautiful.
 Originally posted by Magpie, Tue Mar 29, 2016 7:35 am[
Originally posted by Magpie, Tue Mar 29, 2016 7:35 am[I was looking at the bike on the weekend after reading your post, it is in trouble. I will tell my partner it was all your fault
 Originally posted by Lokiel, Tue Mar 29, 2016 2:39 pm[
Originally posted by Lokiel, Tue Mar 29, 2016 2:39 pm[I've just been informed that "my mate" above is NOT a "Tommy Roundhead" but is actually a young "Intellagama lesueurii (AGAMIDAE) Water Dragon".
http://www.saveourwaterwaysnow.com.au/01_cms/details.asp?ID=913#2417 points out "Can be mistakenly indentified as Tommy Roundhead when juvenile, when the crest is not so obvious.".
I live near a creek and have seen the adult versions which can grow as long and thick as your arm and look quite formidable so I'll be keeping "on the good side" of "my mate".
Originally posted by bruce, Tue Mar 29, 2016 4:03 pm[Are you going to end up gassing yourself?
Do you end up with toxic waste?
Originally posted by Magpie, Tue Mar 29, 2016 4:25 pm[Remember Lokiel said he had a creek down the back
 Originally posted by Lokiel, Tue Mar 29, 2016 4:54 pm[
Originally posted by Lokiel, Tue Mar 29, 2016 4:54 pm[bruce wrote:Are you going to end up gassing yourself?
Do you end up with toxic waste?
Good question regarding toxic waste. I know that nickel plating generates toxic waste due to the chemicals needed but hadn't read anything about aluminium anodizing producing toxic waste other than the acid bath.
I had the garage door up and a fan blowing across the tank to blow the air that way - you could still smell it so it couldn't be good for you in a closed environment.
The sealant pot stunk the house up a bit though so all doors and windows were open.
The dye can be re-used so I stored that for re-use later (~13L) .
I bought swimming pool pH decreaser (acid) AND pH increaser (base) since I knew that there was going to be a lot of strong acid to dispose of.
Once I'd finished, I slowly added the pH increaser into the anodizing tank until it no longer tasted acidic (it had some real "kick" prior to adding the pH increaser, citric acid X 5 - not sure I'd care to try the taste test prior to all that anodising which weakened the electrolyte).
I should've bought some pH paper too but I figured the taste test was good enough - still wasn't keen on emptying the contents onto my garden so it went down the drain.
Originally posted by Lokiel, Wed Mar 30, 2016 1:15 pm[Magpie wrote:Remember Lokiel said he had a creek down the back

Too far "down the back" to lug all those chemicals.
In hindsight, the acid bath would've made a wonderful swimming pool for cane toads!
Originally posted by Magpie, Wed Mar 30, 2016 1:28 pm[Not only do can toads enjoy acid baths, but they react well to a pitching wedge
 Originally posted by MattR, Sat Apr 02, 2016 9:43 pm[
Originally posted by MattR, Sat Apr 02, 2016 9:43 pm[Lokiel wrote:I bought swimming pool pH decreaser (acid) AND pH increaser (base) since I knew that there was going to be a lot of strong acid to dispose of.
Once I'd finished, I slowly added the pH increaser into the anodizing tank until it no longer tasted acidic (it had some real "kick" prior to adding the pH increaser, citric acid X 5 - not sure I'd care to try the taste test prior to all that anodising which weakened the electrolyte).
I should've bought some pH paper too but I figured the taste test was good enough - still wasn't keen on emptying the contents onto my garden so it went down the drain.
So you put it in the creek then? As that is where the storm water will flow to.
Or even worse if it went in the sewer.
Best bet next time ring council, they will tell you best and safest method of disposal of waste.
Even small amounts of chemicals can have pretty bad effects on receiving waters.
Originally posted by Lokiel, Sat Apr 02, 2016 10:26 pm[MattR wrote:Lokiel wrote:I bought swimming pool pH decreaser (acid) AND pH increaser (base) since I knew that there was going to be a lot of strong acid to dispose of.
Once I'd finished, I slowly added the pH increaser into the anodizing tank until it no longer tasted acidic (it had some real "kick" prior to adding the pH increaser, citric acid X 5 - not sure I'd care to try the taste test prior to all that anodising which weakened the electrolyte).
I should've bought some pH paper too but I figured the taste test was good enough - still wasn't keen on emptying the contents onto my garden so it went down the drain.
So you put it in the creek then? As that is where the storm water will flow to.
Or even worse if it went in the sewer.
Best bet next time ring council, they will tell you best and safest method of disposal of waste.
Even small amounts of chemicals can have pretty bad effects on receiving waters.
I think I was quite responsible in my disposal practice, neutralising the acid first and I wasn't using any toxic chemicals like those that are needed for nickel plating.
I doubt many pool owners go to this effort when emptying their swimming pools.
And, if it ended up in the sewer, it was certainly far less toxic than than the sewerage which has an extremely high ammonia content.
Originally posted by MattR, Mon Apr 04, 2016 7:01 pm[But the problem is the chemicals used to alter the pH of the original citric acid, the alkalines used to up the pH can be pretty nasty in themselves and the the natives don't like the nitogens, phosphates, ammonias and so on used in most trade waste stuff.
I have seen some serious environmental damage done by what was though to be innocuous disposal of chemicals down drains in relative small amounts when i worked in the Blue Mountains for Sydney Water.
What we think is alright for the environment can actually be pretty bad for native flora and fauna.
The best bet would have been the garden over the drain, particularly if you have weeds you want to get rid of.